Why Immune Cells Extrude Webs of DNA and Protein
In the mid 2000s, Arturo Zychlinsky at the Max Planck Institute for Infection Biology in Berlin found that mammalian safe cells called neutrophils utilize a chemical called neutrophil elastase (NE) to sever bacterial harmfulness factors. When Zychlinsky and his associates dug further into this protection system, they understood that when enacted by microbes, human neutrophils discharge NE in what, under the magnifying lens, resembled a sinewy structure. This structure ended up being a meshwork of NE, different proteins, and bounteous measures of DNA. In refined human neutrophils, the networks had the option to trap the microscopic organisms that had set off their arrangement, along these lines constraining disease, so Zychlinsky and associates named them neutrophil extracellular snares, or NETs.
The way that neutrophils utilized their atomic material to get pathogens was interesting to immunologists and cell scientists the same. Crafted by the Zychlinsky lab proposed that the arrival of NETs was a functioning procedure, and that the material wasn't just discharged by inactive lysis. This has inspired another line of research committed to describing these special structures, portraying the systems that brief their development, and distinguishing their significance in mammalian science.
As an ever increasing number of scientists join the prospering field, the range of pathogens known to actuate NET discharge from neutrophils has extended from an assortment of microorganisms to organisms and, most as of late, to infections. In any case, it has likewise become evident that NETs can have negative ramifications for the living beings that produce them—by actuating immune system pathways or urging tumor cells to metastasize, for instance.
Today, it is generally acknowledged that NETs have both a defensive and an obsessive effect on the host. In 2012, Mariana Kaplan, presently of the National Institutes of Health, and the University of Tennessee's Marko Radic named NETs a "twofold edged sword of invulnerability" and recommended that sound life forms should firmly control their discharge to limit negative ramifications for the host. The subtleties of NET guideline and capacity are presently an exceptionally dynamic zone of research.
NET fundamentals
Neutrophils are fundamental for resistant protection and counteractive action of microbial abundance. They are exceptionally copious—around 100 billion are created in a human's bone marrow in a solitary day—and they flow in the circulatory system to rapidly invade tissues if the neutrophils identify a microbial danger. Having a place with a class of white platelets called granulocytes, they are portrayed by a cytoplasm pressed with granules containing antimicrobial proteins. Neutrophils can immerse pathogens and afterward intertwine their granules with their phagosomes, which contain the disguised microorganisms. On the other hand, the cells can meld their granules with the plasma layer to discharge antimicrobials to assault extracellular parasites.
Research on neutrophils is entangled by the way that they are brief cells. For example, dissimilar to some other human cell types, neutrophils can't be refined for in excess of a couple of hours, and they are not amiable to quality altering. Hence, despite everything we come up short on a point by point unthinking picture of how precisely NETs are shaped. Early reports affirmed the first theory that NETs don't result from aloof corruption of neutrophils. Later investigations included intricacy by showing that diverse incendiary triggers prompt different pathways that all lead to the arrival of NETs, and that NET discharge doesn't generally bring about lysis of the neutrophil.
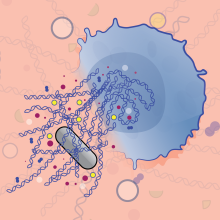
All things considered, most pathways of NET arrangement do murder the insusceptible cell, commonly because of the creation of receptive oxygen species (ROS). Bacterial or contagious pathogens cause neutrophils to actuate kinases that instigate gathering of a catalyst complex called nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. NADPH oxidase at that point delivers a lot of superoxide—a profoundly responsive oxygen exacerbate that conveys an additional electron—during a procedure called the neutrophil oxidative burst. ROS coming about because of the oxidative burst trigger crumbling of a multiprotein complex to discharge dynamic NE, an essential segment of NETs, into the cytoplasm. (See representation on page 45.)
NE at that point relocates to the neutrophil's core, where it severs histones and different proteins to decondense the chromatin. In the long run, the chromatin tops off the whole cell until the cell lyses and expels the NET into the extracellular space, a procedure known as NETosis. We as of late recognized a significant job for the pore-shaping protein gasdermin D in both the atomic development and the lysis forms, despite the fact that the instruments aren't yet clear. In the extracellular space, the networks are thought to trap and execute the activating pathogens.
NETs in safe barrier
NETs' capacity to trap microbial intruders has been exhibited in vitro for an assortment of pathogens. The structures have all the earmarks of being especially significant in resistance against pathogenic growths, recommending NETs may have advanced as an approach to trap enormous life forms that can't be overwhelmed by means of phagocytosis. Be that as it may, an absence of explicit hereditary apparatuses has made it hard to nail down the precise commitment of NETs to antimicrobial barrier in vivo. The majority of the particles that have so far been found to manage NETs are likewise associated with other insusceptible reactions, so a "NET knockout" living being is still past our range.
Notwithstanding these specialized difficulties, analysts are gradually explaining the capacity of NETs in insusceptibility. In 2012, Paul Kubes of the University of Calgary and associates drained NETs in mice with skin contaminations by infusing recombinant DNase 1, a compound that slashes up the DNA spine of NETs after they are discharged. This prompted expanded spread of Staphylococcus aureus from the skin to the circulation system, exhibiting that NETs take part in containing microscopic organisms at the site of section. This trial procedure of NET obliteration by nucleases is additionally utilized normally by pathogenic microscopic organisms as an approach to escape from NETs: Staphylococcus and Streptococcus species both discharge DNases, and exploratory cancellation of the qualities that encode these NET-debasing chemicals in those microorganisms prompts diminished bacterial harmfulness.
The way that neutrophils utilized their atomic material to get pathogens was interesting to immunologists and cell scientists the same. Crafted by the Zychlinsky lab proposed that the arrival of NETs was a functioning procedure, and that the material wasn't just discharged by inactive lysis. This has inspired another line of research committed to describing these special structures, portraying the systems that brief their development, and distinguishing their significance in mammalian science.
As an ever increasing number of scientists join the prospering field, the range of pathogens known to actuate NET discharge from neutrophils has extended from an assortment of microorganisms to organisms and, most as of late, to infections. In any case, it has likewise become evident that NETs can have negative ramifications for the living beings that produce them—by actuating immune system pathways or urging tumor cells to metastasize, for instance.
Today, it is generally acknowledged that NETs have both a defensive and an obsessive effect on the host. In 2012, Mariana Kaplan, presently of the National Institutes of Health, and the University of Tennessee's Marko Radic named NETs a "twofold edged sword of invulnerability" and recommended that sound life forms should firmly control their discharge to limit negative ramifications for the host. The subtleties of NET guideline and capacity are presently an exceptionally dynamic zone of research.
NET fundamentals
Neutrophils are fundamental for resistant protection and counteractive action of microbial abundance. They are exceptionally copious—around 100 billion are created in a human's bone marrow in a solitary day—and they flow in the circulatory system to rapidly invade tissues if the neutrophils identify a microbial danger. Having a place with a class of white platelets called granulocytes, they are portrayed by a cytoplasm pressed with granules containing antimicrobial proteins. Neutrophils can immerse pathogens and afterward intertwine their granules with their phagosomes, which contain the disguised microorganisms. On the other hand, the cells can meld their granules with the plasma layer to discharge antimicrobials to assault extracellular parasites.
Research on neutrophils is entangled by the way that they are brief cells. For example, dissimilar to some other human cell types, neutrophils can't be refined for in excess of a couple of hours, and they are not amiable to quality altering. Hence, despite everything we come up short on a point by point unthinking picture of how precisely NETs are shaped. Early reports affirmed the first theory that NETs don't result from aloof corruption of neutrophils. Later investigations included intricacy by showing that diverse incendiary triggers prompt different pathways that all lead to the arrival of NETs, and that NET discharge doesn't generally bring about lysis of the neutrophil.
All things considered, most pathways of NET arrangement do murder the insusceptible cell, commonly because of the creation of receptive oxygen species (ROS). Bacterial or contagious pathogens cause neutrophils to actuate kinases that instigate gathering of a catalyst complex called nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. NADPH oxidase at that point delivers a lot of superoxide—a profoundly responsive oxygen exacerbate that conveys an additional electron—during a procedure called the neutrophil oxidative burst. ROS coming about because of the oxidative burst trigger crumbling of a multiprotein complex to discharge dynamic NE, an essential segment of NETs, into the cytoplasm. (See representation on page 45.)
NE at that point relocates to the neutrophil's core, where it severs histones and different proteins to decondense the chromatin. In the long run, the chromatin tops off the whole cell until the cell lyses and expels the NET into the extracellular space, a procedure known as NETosis. We as of late recognized a significant job for the pore-shaping protein gasdermin D in both the atomic development and the lysis forms, despite the fact that the instruments aren't yet clear. In the extracellular space, the networks are thought to trap and execute the activating pathogens.
NETs in safe barrier
NETs' capacity to trap microbial intruders has been exhibited in vitro for an assortment of pathogens. The structures have all the earmarks of being especially significant in resistance against pathogenic growths, recommending NETs may have advanced as an approach to trap enormous life forms that can't be overwhelmed by means of phagocytosis. Be that as it may, an absence of explicit hereditary apparatuses has made it hard to nail down the precise commitment of NETs to antimicrobial barrier in vivo. The majority of the particles that have so far been found to manage NETs are likewise associated with other insusceptible reactions, so a "NET knockout" living being is still past our range.
Notwithstanding these specialized difficulties, analysts are gradually explaining the capacity of NETs in insusceptibility. In 2012, Paul Kubes of the University of Calgary and associates drained NETs in mice with skin contaminations by infusing recombinant DNase 1, a compound that slashes up the DNA spine of NETs after they are discharged. This prompted expanded spread of Staphylococcus aureus from the skin to the circulation system, exhibiting that NETs take part in containing microscopic organisms at the site of section. This trial procedure of NET obliteration by nucleases is additionally utilized normally by pathogenic microscopic organisms as an approach to escape from NETs: Staphylococcus and Streptococcus species both discharge DNases, and exploratory cancellation of the qualities that encode these NET-debasing chemicals in those microorganisms prompts diminished bacterial harmfulness.
Email us at: clinicalmicrobiology@pulsusgathering.com



Comments
Post a Comment